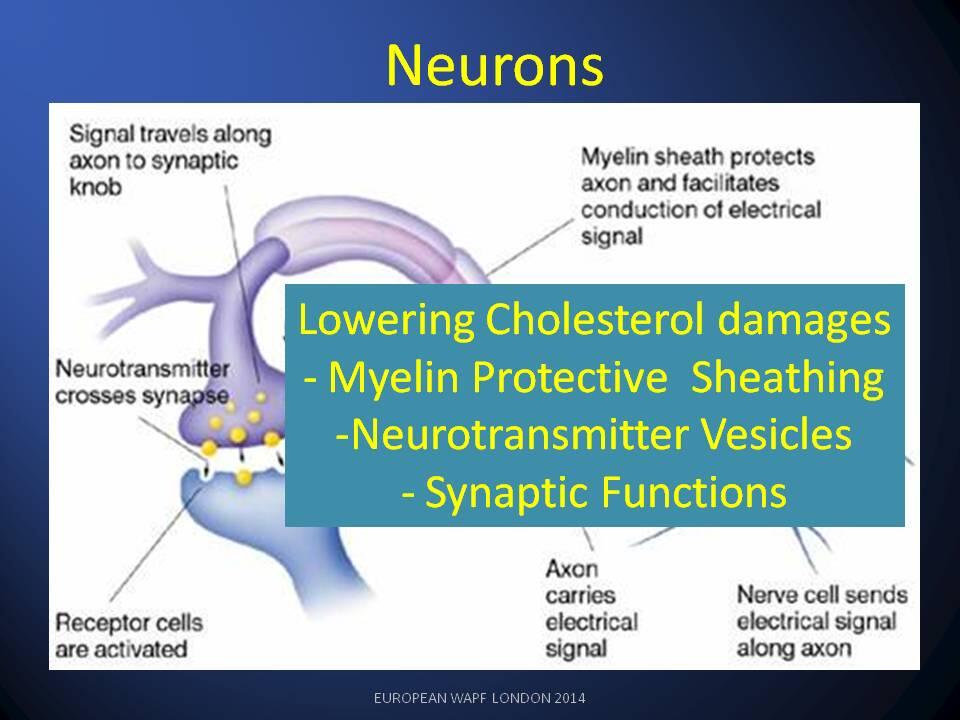
productive over the long-term.
Tag Archives: adr
Reply
diabetes [9]. The underlying mechanisms of the
potential adverse effects of statins on carbohydrate
homeostasis are complex [10] and might be related
to the lipophilicity of the statin [11]. Indeed,
retrospective analysis of the West of Scotland
Coronary Prevention Study (WOSCOPS) revealed
that 5 years of treatment with pravastatin reduced
diabetes incidence by 30% [12]. The authors
suggested that although lowering of trigliceride
levels could have influenced diabetes incidence,
other mechanisms such as anti-inflammatory action
might have been involved; however, in the
multivariate Cox model, baseline total cholesterol
did not predict the development of diabetes [12].
Furthermore, pravastatin did not decrease diabetes
incidence in the LIPID trial which included glucose-
intolerant patients [13]. On the other hand, in the
JUPITER trial (Justification for the Use of Statins in
Prevention: an Intervention Trial Evaluating
Rosuvastatin), which studied apparently healthy
persons without hyperlipidemia but with elevated
high-sensitivity C-reactive protein levels [14], the
risk of diabetes was increased by a factor of 1.25
[95% confidence interval (CI), 1.05 to 1.51] among
individuals receiving rosuvastatin 20 mg daily with
respect to placebo. Strikingly, among persons
assigned to rosuvastatin, the median low density
lipoprotein (LDL) cholesterol level at 12 months was
55 mg per deciliter [interquartile range, 44 to 72
(1.1 to 1.9)].
It is intriguing that salutary lifestyle measures,
which might exert their beneficial action through
an anti-inflammatory mechanism without a strong
cholesterol-lowering effect, beyond reducing
cardiovascular events and total mortality, reduce
also the risk of diabetes and other chronic
degenerative diseases. This fact may represent
a ‘justification’ not to use a drug in low-risk primary
prevention populations: lowering cholesterol at the
expense of increasing diabetes might be counter-
References
9. Sukhija R, Prayaga S, Marashdeh M, et al. Effect of statins
on fasting plasma glucose in diabetic and nondiabetic
patients. J Investig Med 2009; 57: 495-9.
10. Szendroedi J, Anderwald C, Krssak M, et al. Effects of high-
dose simvastatin therapy on glucose metabolism and
ectopic lipid deposition in nonobese type 2 diabetic
patients. Diabetes Care 2009; 32: 209-14.
11. Ishikawa M, Okajima F, Inoue N, et al. Distinct effects of
pravastatin, atorvastatin, and simvastatin on insulin
secretion from a beta-cell line, MIN6 cells. J Atheroscler
Thromb 2006; 13: 329-35.
12. Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the
development of diabetes mellitus: evidence for
a protective treatment effect in the West of Scotland
Coronary Prevention Study. Circulation 2001; 103: 357-62.
13. K
eech A, Colquhoun D, Best J, et al.; LIPID Study Group.
Secondary prevention of cardiovascular events with long-
term pravastatin in patients with diabetes or impaired
fasting glucose: results from the LIPID trial. Diabetes Care
2003; 26: 2713-21.
14. Ridker PM, Danielson E, Fonseca FA, et al.; JUPITER Study
Group. Rosuvastatin to prevent vascular events in men
and women with elevated C-reactive protein. N Engl J Med
2008; 359: 2195-207.
Link